New Invention Helps Protect Healthcare Workers During COVID-19 Pandemic
By Sharon Holland
If necessity and innovation are the driving forces behind invention, then the “COVID-19 Airway Management Isolation Chamber,” or CAMIC, is the perfect creation. The device, conceived, designed, built and tested by Military Health System and the Army’s Telemedicine and Advanced Technology Research Center (TATRC) personnel, may be the answer to protecting healthcare workers from COVID-19 and other viruses during patient care.
CAMIC, which recently received approval from the FDA for emergency use, is currently the first and only FDA-approved adjunct personal protective equipment (PPE) of its kind with a negative pressure vacuum validated to be effective in containing and reducing aerosols and airborne particles. (https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization#covidppe)
The idea for the device came from Army Maj. (Dr.) Steven Hong, an assistant professor of Surgery at the Uniformed Services University (USU) and chief of Head and Neck Surgical Oncology and Reconstructive Surgery at Walter Reed National Military Medical Center (WRNMMC). As he watched the COVID-19 crisis unfold across the world, Hong saw how it was overwhelming the healthcare systems in Italy and New York. He knew that COVID-19 was very contagious and healthcare workers, especially within his specialty of head and neck surgery where they are often exposed to nasal and respiratory droplets, seemed to be more vulnerable.
“The news of the lack of adequate PPE, especially in New York at that time, was alarming and we realized there could be an adjunct PPE that could better protect healthcare workers,” Hong said.
“Dr. Hong asked if I was interested in developing a device to more safely perform tracheostomy on patients in light of the ongoing COVID-19 pandemic. Initial reports in China documented a high rate of transmission to the OR team from procedures involving the nasal cavity and airway. Given the sheer number of intubations (and likely need for tracheostomy) estimated, we wanted to make something to decrease the risk to our team and OR staff in the event of a tracheostomy,” said Blood.
Krivda’s USU medical school class was largely sidelined from patient care when their clerkship rotations were suspended due to the COVID-19 pandemic. Like many of his classmates, he was looking for ways to stay involved. He had talked with Hong throughout his first year of medical
school about getting involved in research, so when Krivda returned to campus, his timing was impeccable.
“I do not have an engineering background, and if you told me a month ago that I would be an inventor on a U.S. Army patent, I would not have believed you. But when there is a serious problem to be addressed, like the one posed by coronavirus transmission to healthcare workers, I’m more than willing to work tirelessly to find a solution, and being a part of this team gave me the opportunity to do just that,” he said.
After the initial design and prototypes were made, the team realized that while the CAMIC would function well for tracheostomy, its true use would be for intubation and possibly for nebulization and for non-invasive ventilation to decrease the need for ventilator use and ICU requirements.
“We wanted to develop a 3D-printed chamber to contain aerosolization from airway procedures. Initially, we wanted to go from the top of the patient’s head to the mid-chest. Quickly this was noted to be too bulky and was shortened to go to the top of the shoulders. After identifying that the chamber could have broad implications both at home and abroad for the care of COVID-19 patients, the design drastically changed,” said Blood.
PVC piping was selected for the chamber’s frame because of its strength, availability, and cost, but an outer liner was more difficult to design, Blood said. “While testing out our design in the OR, we discovered a polyethylene bag that worked great as a chamber liner and we have been moving forward since then.” Cost of the first prototype? About $15, he said.
The team worked with nurse anesthetists and anesthesiologists for proof-of-concept testing. USU neuroscience graduate and Army certified registered nurse anesthetist (CRNA) Lt. Col. Robert Long, and USU CRNA student Navy Lt. Caroline Mosher, were among the anesthesia personnel testing the chamber’s viability at WRNMMC.
“They flat told us that the widely available clear acrylic intubation box design made it difficult to use given the rigid hole placement and the fact that the top of the box is contacted when performing an intubation. It was at that time we tried to come up with a better solution,” said Blood.
“What really demonstrated the absolute need to push the design was when we performed our cough test using fluorescent powder. The CAMIC completely contained the particles while the other option, the clear acrylic box, allowed egress of the powder into the room in alarming amounts. It was easy to see how many people in the OR or ER might get infected during airway procedures,” said Blood.
Although it is intended to keep aerosol particulate isolated inside the chamber, surgeons can still use the device while performing procedures. Holes in the side of the chamber provide access to the patient. These holes are easily sealed with tape if access is no longer needed, and testing suggests that they are able to contain and evaluate droplet and aerosol particulate in a very efficient manner. The CAMIC has a drawstring to close over the chest for an adequate seal, and while it was designed to decrease risk to the team and OR staff, it does not decrease the need for full PPE. The CAMIC is equipped with an in-frame suction and air inlet providing a laminar flow environment which is the key to the device. The proof-of-concept intubation using the CAMIC and further testing with both ear and airway cases demonstrated the ability to work through the CAMIC effectively. The team has yet to perform a tracheostomy. Initially, they said, it may take a little longer than normal for routine cases but they feel the decreased aerosolization secondary to surgical manipulation and decreased risk to the team and OR staff is worth the extra few minutes.
“It has been a whirlwind of ideas and countless hours of validation and testing. We have been able to go from concept to design, build, validation, redesign, rebuild, revalidation x 10, IRB process, patent process, and FDA Emergency Use Authorization clearance in less than six weeks. It has been truly remarkable to see the dedication and motivation of my team,” said Hong. “I spent the last couple of years in one of the most innovative environments, Silicon Valley, and the mantra that this team has carried throughout this process reminds me of that time.”
Hong said the fact that they were able to get this far in a process that typically takes much longer is largely because of the people behind the scenes.
“To me, it was a combination of three things. First, obviously there was the need for some additional type of PPE beyond what was already available due to the extensive shortages created by the uncertainty and chaos created by COVID-19. As they say, ‘necessity is the mother of all inventions.’ Second, the conducive environment that was facilitated by our leaders and the institution as a whole was critical. From the IRB to our direct leadership, everyone went out of their way to guide us and assist in accelerating this entire process. Their encouragement and support in this process cannot be overstated. And, most importantly, it was just the sheer willpower of our team members, especially our junior members, Capt. Tim Blood, Capt. Nate Perkins, Capt. Paul Wistermayer, and 2nd Lt. Joe Krivda. They probably have worked harder in the last three to four weeks than they had in a long time,” he said.
Blood echoes that sentiment. “We have had an extremely dedicated team at the Walter Reed, Belvoir and Madigan Otolaryngology departments. We all have different strengths and we are contributing wherever we can. Multiple things needed to occur simultaneously from patent application, IRB submission, FDA application, testing, and writing for the papers; we worked well together. We have also had the blessing of our clinic chief to continue with testing and development of the project. We wanted to go through the proper channels to provide the greatest benefit to patients in the future. In the midst of the pandemic, healthcare workers are doing anything they can to protect themselves to allow treatment of more patients. We wanted to help with that goal any way possible. Right now multiple Defense Health Agency and civilian sites are using the CAMIC as an urgently needed low tech solution to protect healthcare workers during the pandemic.”
Although the CAMIC was approved for emergency use by the FDA, the device will still need to go through a regular approval process in the future. In the meantime, the U.S. Army Medical Research and Development Command is seeking manufacturing partners through its technology transfer program as a long-term solution, Hong said.
“Much like the flu of 1918, SARS, MERS and now COVID-19, respiratory pandemics will continue to occur. Additionally, the CAMIC could potentially be adaptable for any respiratory infection to decrease risk to healthcare workers. Active TB, and all sorts of easily transmissible respiratory infections occurred before COVID-19 and will continue to occur,” he continued. “Additionally, we have been looking at making the CAMIC capable of being used for transport and evacuation purposes for both first responders in the U.S. and for transport outside the country. We hope that it will be used in the near future to help protect our healthcare workers, alleviate the PPE shortages, and potentially help patients avoid early intubations by being able to safely receive nebulization treatments or BIPAP without fear of spreading aerosols widely.”
The device is currently being used at Walter Reed, Fort Belvoir, Fort Hood, Fort Bliss, Madigan, U.S. Central Command, Kapiolani Hospital in Honolulu, and INOVA Alexandria and Fairfax. Furthermore, says Krivda, the CAMIC’s simple design and availability of parts allows it to be easily constructed at practically any hospital in the world.
“Although we are dealing with the COVID-19 pandemic now, I am absolutely certain that the military will be called on in the future to battle other pandemics,” said Hong. “One of our main objectives will be to develop a more operational-friendly CAMIC that could be used downrange or for transport of infectious patients. Dr. Douglas Ruhl is currently leading this next-step effort and we look forward to the next generation of innovations.”
Blood agrees that their invention is important to the military’s readiness mission.
“The goal of the CAMIC is to provide additional protection for frontline healthcare workers to ensure decreased nosocomial infections and overall healthier hospital staff. This directly improves the deployability of medics, corpsman, nurses, physicians, and others. While we have not done human testing with non-invasive positive pressure ventilation (NIPPV)/nebulization yet, we feel our data suggest that our device might allow NIPPV/nebs to be used safely. The ability to safely provide NIPPV and avoid endotracheal intubation would be a game changer and this would provide the capability to conserve vital ventilators and ICU space, thus freeing additional resources that can be used in other aspects of military readiness,” he said.
The entire team credits the support from their department and command leadership and other Military Health System colleagues as key to their success in going from concept to emergency use in rapid fashion.
“In a miniscule amount of time, this team has created, tested, and proven an idea that can and will go on to have an immense impact – I am incredibly grateful to have been a part of it,” said Krivda.
If necessity and innovation are the driving forces behind invention, then the “COVID-19 Airway Management Isolation Chamber,” or CAMIC, is the perfect creation. The device, conceived, designed, built and tested by Military Health System and the Army’s Telemedicine and Advanced Technology Research Center (TATRC) personnel, may be the answer to protecting healthcare workers from COVID-19 and other viruses during patient care.
CAMIC, which recently received approval from the FDA for emergency use, is currently the first and only FDA-approved adjunct personal protective equipment (PPE) of its kind with a negative pressure vacuum validated to be effective in containing and reducing aerosols and airborne particles. (https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization#covidppe)
The idea for the device came from Army Maj. (Dr.) Steven Hong, an assistant professor of Surgery at the Uniformed Services University (USU) and chief of Head and Neck Surgical Oncology and Reconstructive Surgery at Walter Reed National Military Medical Center (WRNMMC). As he watched the COVID-19 crisis unfold across the world, Hong saw how it was overwhelming the healthcare systems in Italy and New York. He knew that COVID-19 was very contagious and healthcare workers, especially within his specialty of head and neck surgery where they are often exposed to nasal and respiratory droplets, seemed to be more vulnerable.
“The news of the lack of adequate PPE, especially in New York at that time, was alarming and we realized there could be an adjunct PPE that could better protect healthcare workers,” Hong said.
“Dr. Hong asked if I was interested in developing a device to more safely perform tracheostomy on patients in light of the ongoing COVID-19 pandemic. Initial reports in China documented a high rate of transmission to the OR team from procedures involving the nasal cavity and airway. Given the sheer number of intubations (and likely need for tracheostomy) estimated, we wanted to make something to decrease the risk to our team and OR staff in the event of a tracheostomy,” said Blood.
Krivda’s USU medical school class was largely sidelined from patient care when their clerkship rotations were suspended due to the COVID-19 pandemic. Like many of his classmates, he was looking for ways to stay involved. He had talked with Hong throughout his first year of medical
school about getting involved in research, so when Krivda returned to campus, his timing was impeccable.
“I do not have an engineering background, and if you told me a month ago that I would be an inventor on a U.S. Army patent, I would not have believed you. But when there is a serious problem to be addressed, like the one posed by coronavirus transmission to healthcare workers, I’m more than willing to work tirelessly to find a solution, and being a part of this team gave me the opportunity to do just that,” he said.
After the initial design and prototypes were made, the team realized that while the CAMIC would function well for tracheostomy, its true use would be for intubation and possibly for nebulization and for non-invasive ventilation to decrease the need for ventilator use and ICU requirements.
“We wanted to develop a 3D-printed chamber to contain aerosolization from airway procedures. Initially, we wanted to go from the top of the patient’s head to the mid-chest. Quickly this was noted to be too bulky and was shortened to go to the top of the shoulders. After identifying that the chamber could have broad implications both at home and abroad for the care of COVID-19 patients, the design drastically changed,” said Blood.
PVC piping was selected for the chamber’s frame because of its strength, availability, and cost, but an outer liner was more difficult to design, Blood said. “While testing out our design in the OR, we discovered a polyethylene bag that worked great as a chamber liner and we have been moving forward since then.” Cost of the first prototype? About $15, he said.
The team worked with nurse anesthetists and anesthesiologists for proof-of-concept testing. USU neuroscience graduate and Army certified registered nurse anesthetist (CRNA) Lt. Col. Robert Long, and USU CRNA student Navy Lt. Caroline Mosher, were among the anesthesia personnel testing the chamber’s viability at WRNMMC.
“They flat told us that the widely available clear acrylic intubation box design made it difficult to use given the rigid hole placement and the fact that the top of the box is contacted when performing an intubation. It was at that time we tried to come up with a better solution,” said Blood.
“What really demonstrated the absolute need to push the design was when we performed our cough test using fluorescent powder. The CAMIC completely contained the particles while the other option, the clear acrylic box, allowed egress of the powder into the room in alarming amounts. It was easy to see how many people in the OR or ER might get infected during airway procedures,” said Blood.
Although it is intended to keep aerosol particulate isolated inside the chamber, surgeons can still use the device while performing procedures. Holes in the side of the chamber provide access to the patient. These holes are easily sealed with tape if access is no longer needed, and testing suggests that they are able to contain and evaluate droplet and aerosol particulate in a very efficient manner. The CAMIC has a drawstring to close over the chest for an adequate seal, and while it was designed to decrease risk to the team and OR staff, it does not decrease the need for full PPE. The CAMIC is equipped with an in-frame suction and air inlet providing a laminar flow environment which is the key to the device. The proof-of-concept intubation using the CAMIC and further testing with both ear and airway cases demonstrated the ability to work through the CAMIC effectively. The team has yet to perform a tracheostomy. Initially, they said, it may take a little longer than normal for routine cases but they feel the decreased aerosolization secondary to surgical manipulation and decreased risk to the team and OR staff is worth the extra few minutes.
“It has been a whirlwind of ideas and countless hours of validation and testing. We have been able to go from concept to design, build, validation, redesign, rebuild, revalidation x 10, IRB process, patent process, and FDA Emergency Use Authorization clearance in less than six weeks. It has been truly remarkable to see the dedication and motivation of my team,” said Hong. “I spent the last couple of years in one of the most innovative environments, Silicon Valley, and the mantra that this team has carried throughout this process reminds me of that time.”
Hong said the fact that they were able to get this far in a process that typically takes much longer is largely because of the people behind the scenes.
“To me, it was a combination of three things. First, obviously there was the need for some additional type of PPE beyond what was already available due to the extensive shortages created by the uncertainty and chaos created by COVID-19. As they say, ‘necessity is the mother of all inventions.’ Second, the conducive environment that was facilitated by our leaders and the institution as a whole was critical. From the IRB to our direct leadership, everyone went out of their way to guide us and assist in accelerating this entire process. Their encouragement and support in this process cannot be overstated. And, most importantly, it was just the sheer willpower of our team members, especially our junior members, Capt. Tim Blood, Capt. Nate Perkins, Capt. Paul Wistermayer, and 2nd Lt. Joe Krivda. They probably have worked harder in the last three to four weeks than they had in a long time,” he said.
Blood echoes that sentiment. “We have had an extremely dedicated team at the Walter Reed, Belvoir and Madigan Otolaryngology departments. We all have different strengths and we are contributing wherever we can. Multiple things needed to occur simultaneously from patent application, IRB submission, FDA application, testing, and writing for the papers; we worked well together. We have also had the blessing of our clinic chief to continue with testing and development of the project. We wanted to go through the proper channels to provide the greatest benefit to patients in the future. In the midst of the pandemic, healthcare workers are doing anything they can to protect themselves to allow treatment of more patients. We wanted to help with that goal any way possible. Right now multiple Defense Health Agency and civilian sites are using the CAMIC as an urgently needed low tech solution to protect healthcare workers during the pandemic.”
 |
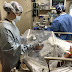
| Capt. (Dr.) Timothy Blood goes through the procedures for performing a tracheostomy using the initial CAMIC prototype, with co-inventor Maj. (Dr.) Steven Hong as the "patient." (Courtesy photo) |
Although the CAMIC was approved for emergency use by the FDA, the device will still need to go through a regular approval process in the future. In the meantime, the U.S. Army Medical Research and Development Command is seeking manufacturing partners through its technology transfer program as a long-term solution, Hong said.
“Much like the flu of 1918, SARS, MERS and now COVID-19, respiratory pandemics will continue to occur. Additionally, the CAMIC could potentially be adaptable for any respiratory infection to decrease risk to healthcare workers. Active TB, and all sorts of easily transmissible respiratory infections occurred before COVID-19 and will continue to occur,” he continued. “Additionally, we have been looking at making the CAMIC capable of being used for transport and evacuation purposes for both first responders in the U.S. and for transport outside the country. We hope that it will be used in the near future to help protect our healthcare workers, alleviate the PPE shortages, and potentially help patients avoid early intubations by being able to safely receive nebulization treatments or BIPAP without fear of spreading aerosols widely.”
The device is currently being used at Walter Reed, Fort Belvoir, Fort Hood, Fort Bliss, Madigan, U.S. Central Command, Kapiolani Hospital in Honolulu, and INOVA Alexandria and Fairfax. Furthermore, says Krivda, the CAMIC’s simple design and availability of parts allows it to be easily constructed at practically any hospital in the world.
“Although we are dealing with the COVID-19 pandemic now, I am absolutely certain that the military will be called on in the future to battle other pandemics,” said Hong. “One of our main objectives will be to develop a more operational-friendly CAMIC that could be used downrange or for transport of infectious patients. Dr. Douglas Ruhl is currently leading this next-step effort and we look forward to the next generation of innovations.”
Blood agrees that their invention is important to the military’s readiness mission.
“The goal of the CAMIC is to provide additional protection for frontline healthcare workers to ensure decreased nosocomial infections and overall healthier hospital staff. This directly improves the deployability of medics, corpsman, nurses, physicians, and others. While we have not done human testing with non-invasive positive pressure ventilation (NIPPV)/nebulization yet, we feel our data suggest that our device might allow NIPPV/nebs to be used safely. The ability to safely provide NIPPV and avoid endotracheal intubation would be a game changer and this would provide the capability to conserve vital ventilators and ICU space, thus freeing additional resources that can be used in other aspects of military readiness,” he said.
The entire team credits the support from their department and command leadership and other Military Health System colleagues as key to their success in going from concept to emergency use in rapid fashion.
“In a miniscule amount of time, this team has created, tested, and proven an idea that can and will go on to have an immense impact – I am incredibly grateful to have been a part of it,” said Krivda.