USU Professor Researches Novel Pain Management Therapy Using Light
By Vivian Mason
Dr. Juanita Anders says we should think about wavelengths of light in a similar capacity to how we think of any pharmaceutical with the potential to treat pain or other disorders. As an internationally recognized expert in photobiomodulation (PBM) with more than 20 years of research under her belt, Anders, a professor of Anatomy, Physiology, and Genetics, as well as a professor of Neuroscience at the Uniformed Services University (USU), has played a major role in establishing the validity of light as clinically beneficial at specific wavelengths and dosages.
The Anders Research Lab is currently focused on achieving a primary goal of military medical research: the development of a novel, non-pharmaceutical therapy for pain management. The lab is supported by funding from a USU Pain Research and Management grant and a Transforming Technology for the Warfighter grant, enabling USU faculty and investigators to be at the forefront of Clinical and Rehabilitative Medicine research.
Introduction to Photobiomodulation
So, how exactly does light have the ability to be clinically beneficial? And what is photobiomodulation?
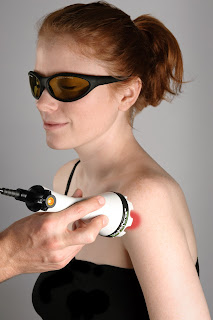 |
| Illustration of light application for temporal mandibular joint pain. (Photo courtesy of Brian Pryor, Ph.D., Vice President, Lightforce@DJO Global) |
She defines PBM as the mechanism by which non-ionizing optical radiation in the visible and near-infrared spectral range is absorbed by endogenous chromophores to elicit photophysical and photochemical effects at various biological scales. Simply put, PBM describes the process in which specific molecules in the human body (endogenous chromophores) are able to absorb light at certain wavelengths, increasing cellular metabolism.
Photobiomodulation therapy (PBMT) is the use of non-ionizing light, such as lasers and LEDs, at those wavelengths—primarily red or near-infrared light—to trigger PBM, increasing cellular metabolism to induce changes in a disease or injury state with the potential to produce positive clinical and physiological benefits, such as tissue repair or decreased pain and inflammation.
And though there is still plenty to learn, PBMT has a history extending back to the 1960s. PBMT, previously known as low-level laser therapy, came about with the discovery of the ruby laser by Theodore Maiman, an American engineer and physicist. In 1967, Dr. Endre Mester, a Hungarian physician and pioneer of laser medicine, conducted an experiment to try and cure malignant tumors in mice.
Benefits of PBMT
In his 1967 experiment, Mester used low-level laser doses on mice. The laser dosage didn't cure the cancer, but it did trigger hair growth and better wound healing. Numerous studies have shown that PBMT provides significant recovery and healing of injured, damaged, and infected tissue, and addresses various health issues.
PBMT brings three major benefits: improved wound tissue regeneration, reduced inflammation, and improved analgesic effect. PBMT can also boost collagen production, improve mood and sleep, treat brain disorders, diseases of the joints, degenerative disc disease, fractures, lymphoedema, myofascial pain, nerve injury and neuropathies, soft tissue injuries, and tendinopathies.
And even with all of those remarkable outcomes, the Anders Research Lab continues to discover new, effective uses for PMBT.
The Anders Research Lab
In their research, the Anders Lab uses various wavelengths of light at varying dosages to characterize how tissues and cells will respond at the molecular and functional levels. Learning how the tissues and cells respond helps them optimize the dose of each wavelength to induce different results based on the need of the patient.
 |
| Dr. Juanita Anders, professor of Anatomy, Physiology, and Genetics, and professor of Neuroscience at USU's School of Medicine. (Photo credit: Dr. Juanita Anders, USU) |
For example, Anders’ team found that high-dose PBMT applied through the skin reduced pain in amounts similar to a peripheral nerve lidocaine injection (a local anesthetic).
“We established that near infrared light (810 nanometers, or nm) at the optimized treatment parameters can block pain transmission as measured by electrophysiology and functional/behavioral tests without blocking α-motor neuron action potentials or large myelinated sensory neurons,” Anders explains. “These data are the first demonstrations of differential nerve blockade using PBM therapy (PBMT).”
In addition to the device, Anders Lab researchers are interested in studying the disruption of the transport of pain. When researchers put light on certain kinds of nerves, they were able to block transmission of pain and temperature sensation that’s carried in the small, non-myelinated and unmyelinated neurons without affecting the large neurons that control motor function. The Anders Lab was the first to demonstrate this selective blocking of various modalities within one nerve.
The Lab also emphasizes the necessity of examining how light penetrates the body, which Anders says is essential to treating deeper issues.
“If I’m treating skin conditions, such as burns or wounds, I can optimize a wide range of wavelengths because transmission to the skin is not a challenge. But, if I want to treat tissues deep in the body, then I need to use a wavelength of light that can penetrate to the target tissue.”
The wavelength determines the depth to which the light photons will go into the body. Then, it’s the power of the laser that determines the number of photons that will reach the target tissue. Anders and her team found that the wavelength with the deepest penetration in human and other mammalian tissue was 810nm, which has now become the widely accepted standard in the field.
“We found that there was a group of wavelengths from about 790–820 nm, where the photons could penetrate to the depths of the spinal cord.”
Anders explains that her lab showed that, when delivered transcutaneously (through the skin), light altered the gene expression of many cell types including neurons and glia.
“It improved recovery after spinal cord injury by altering the immune response and the secondary injury cascade,” she said, noting the “cascade” of changes that begins within just a few hours after a spinal cord injury and can continue months past the initial injury.
The Future
The goal, Anders says, is to develop a device that could be used in remote battlefield areas to block pain transmission utilizing varying dosage treatments.
“Our recommendation is if someone’s in severe pain, block the pain using high-dose treatment and then use a lower dose treatment to continue to change the phenotypic expression of the injured neurons and decrease inflammation.”
But why stop at the battlefield? Anders explains that the device, which the Lab is now primed to design and prototype, could be used along the entire healthcare spectrum with incredible implications.
“Pain is a huge clinical challenge, and opioid drug abuse is a serious complication,” Anders says. “We could use light effectively to control a person’s pain and decrease opioid use. This is very exciting to me just thinking about being able to stop people’s pain. As a non-pharmaceutical treatment, it can benefit people and be quite effective.”